CiA® 412: CANopen profiles for medical devices
The CiA 412 profiles specify the CANopen CC (classic) interfaces for automatic X-ray collimators (CiA 412-2) and dose measurement systems (CiA 412-6). Profiles for X-ray generators (CiA 412-3), patient tables (CiA 412-4), and X-ray stands (CiA 412-5) are also under consideration. Using standardized CANopen interfaces, device manufacturers can supply diverse markets with medical devices that implement the same electronic interface according to CiA 412, and simply vary the application software as needed. System designers can choose between CANopen devices from different manufacturers that offer the same profile-compliant functionality. Off-the-shelf CANopen tools can be used for the development, analysis, and maintenance of these devices.
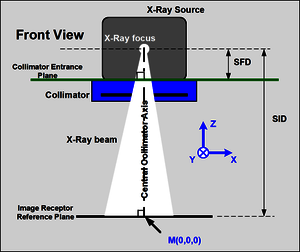
A collimator (see CiA 412-2) has three basic functions for which the profile specifies the appropriate configuration, application, and diagnostic parameters. The main function limits (or collimates) the X-ray beam (e.g., rectangular collimation) emitted by an X-ray source (X-ray tube) to a specified receptor format. Filters may be applied to influence the spectral characteristics of the X-ray beam. The visual simulation of the X-ray beam is the third specified functionality. Some automatic X-ray collimators support local control functionality. The specified collimator functionalities (X, Y, s, ω, D) can be controlled either in position or velocity mode. Devices compliant with this profile are required to support emergency messages (see CiA 301). The specified device errors are categorized into warnings, recoverable errors, and non-recoverable errors.
The collimator device FSA (finite state automaton) specifies the application behavior and corresponding state transitions of the collimators. Since a collimator can be used with local control even when CAN communication is faulty, the communication FSA (see CANopen CC, CiA 301) and the collimator FSA are loosely coupled. The profile also specifies a coordinate FSA applicable to symmetric rectangular collimation sets, quadrangle collimation sets, circular collimation sets, and spatial filter sets. The third specified FSA (homogeneous filter FSA) has the same states as the coordinate FSA but with different definitions for some states. Additionally, the X-ray visualization FSA is specified. The profile provides use case scenarios, such as coordinate motion between specified limits and changes in the SID (source-image distance) value. CiA 412-2 specifies one RPDO (receive process data object) containing the collimator command and the target X-Y position value, and one TPDO (transmit process data object) providing the collimator state and the actual X-Y position value.
The CANopen dose measurement system (see CiA 412-6) measures the X-ray dose and the dose area product. It also measures the dose area product rate, dose rate, RD (reference distance) entrance/skin dose, RD entrance/skin dose rate, MD (measured distance) entrance/skin dose, MD entrance/skin dose rate, irradiation time, chamber temperature, and air pressure values. The actual measured values (field values) are converted into values with a real physical dimension (process values). The profile specifies all required objects to perform this conversion and represent the mentioned values in a standardized manner. Additionally, CiA 412-6 introduces an FSA for dose measurement systems. The profile specifies one RPDO and two TPDOs: RPDO1 transfers the control word, TPDO1 transfers the status word, and TPDO2 transfers the current process value.
| Title | Details | Published Size |
Status Action |
|---|---|---|---|
|
|
ScopeThis specification specifies the CANopen application layer. This includes the data types, encoding rules and object dictionary objects as well as the CANopen communication services and protocols. In addition, this specification specifies the CANopen network management services and protocols. This specification specifies the CANopen communication profile, e.g. the physical layer, the pre- defined communication object identifier connection set, and the content of the Emergency, Time- stamp, and Sync communication objects. |
2011-02-21 3 MB | PAS Login |
|
|
ScopeThis specification defines the CANopen device profile for dose measurement systems. A prerequisite for the conformity to this CANopen device profile is conformity with the CANopen communication profile. Additionally, in the case that the module is programmable conformance to the framework for programmable CANopen devices is required. It is recommended to consulted these specifications in parallel to this device profile specification. |
2008-08-27 2 MB | PAS Login |
|
|
ScopeThe CANopen profiles for medical devices includes several parts: • Part 1 describes general definitions; • Part 2 defines the profile for automatic X-ray collimators; • Part 3 defines the profile for X-ray generators; • Part 4 defines the profile for patient tables; • Part 5 defines the profile for X-ray stands. Devices compliant to these profiles use communication techniques, which conforms to those described in the CANopen communication profile (CiA Draft Standard DS-301, /2/). In addition, medical devices and sub-systems may use communication techniques, which conform to those described in the framework for programmable CANopen Devices (CiA Draft Standard Proposal DSP-302). These specifications should be consulted in parallel to these device profile specifications. |
2005-12-31 167 KB | PAS Login |
|
|
ScopeThis document represents the CANopen device profile for generic X-ray collimators, and as such describes the generic subset of collimator functionality. A prerequisite for the conformity to this CANopen device profile is conformity with the CANopen communication profile (CiA Draft Standard DS 301). Additionally, in the case that the module is programmable it must conform to the Framework for programmable CANopen devices (CiA Draft Standard Proposal DSP 302). These specifications should be consulted in parallel to this device profile specification. |
2005-12-31 1 MB | PAS Login |